There are two types of B cell activation, requiring or not interaction with CD4+ T cells:
- thymus-dependent activation
This is the conventional pathway of B cell activation described in this chapter. It requires interaction with a previously activated CD4+ T cell. - Thymus-independent activation
This pathway mainly concerns B cells in the marginal zone of the spleen. It does not require interaction with CD4+T cells , and sometimes even no recognition by the BCR. The antigens leading to these activations are very generally microbial and lead to the genesis of short-lived IgM plasmablasts , but do not lead to the synthesis of memory B cells or class switching.
Thymus-independent activation mechanisms are not described here because they are poorly represented in alloreactivity.
Actors involved
Mechanism
Where?
In the B cell zone of the secondary lymphoid organs, which constitutes a zone of concentration of antigens:
- In the lymph nodes: the cortical zone
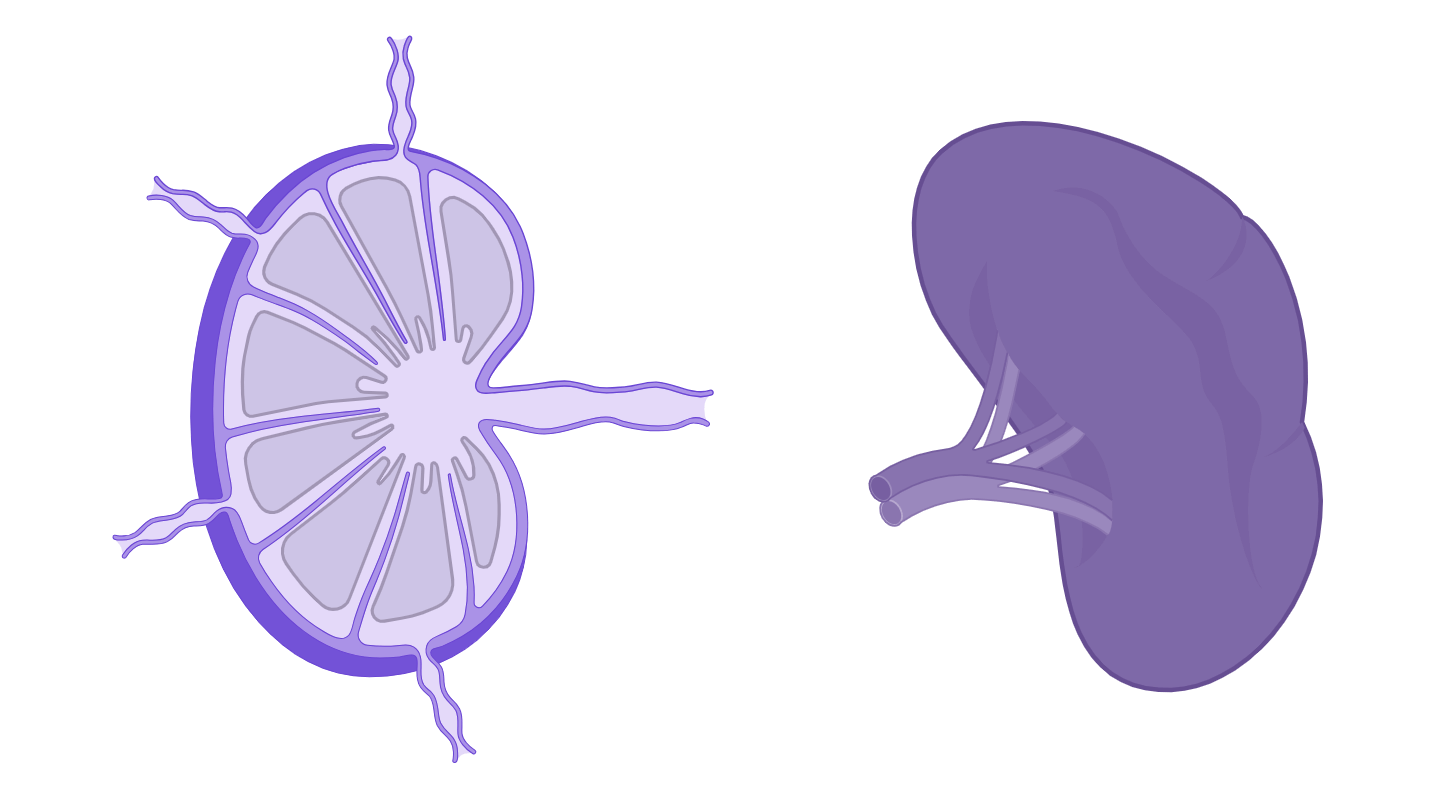
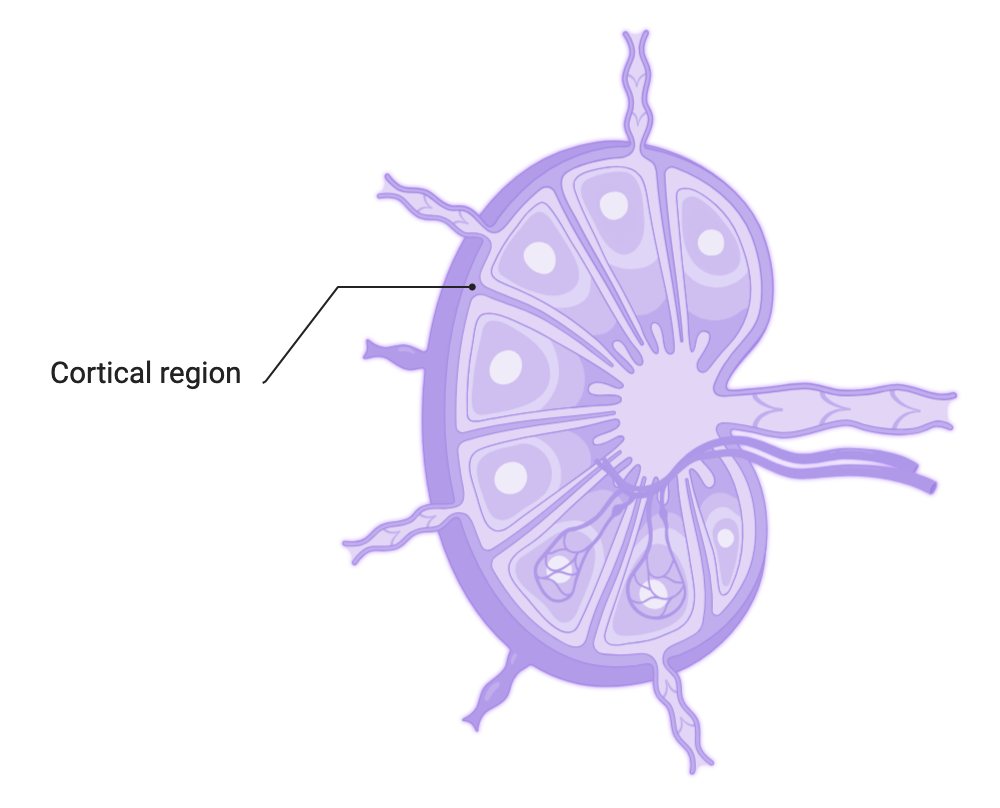 Organisation of a lymph node
Organisation of a lymph node- In the spleen : within the white pulp, in the primary and secondary follicles
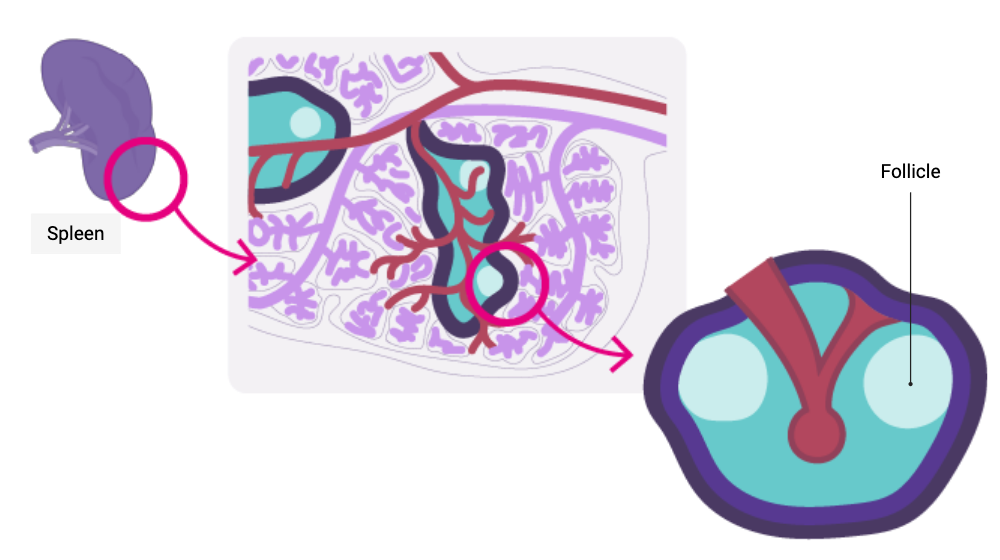 Organisation of the white pulp of the spleen
Organisation of the white pulp of the spleenWhen?
After having undergone central selection in the bone marrow, then peripheral in the blood and in the spleen in order to eliminate auto-reactive B cells (see B cell ontogeny), B cells circulate in the bloodstream and enter secondary lymphoid organs.
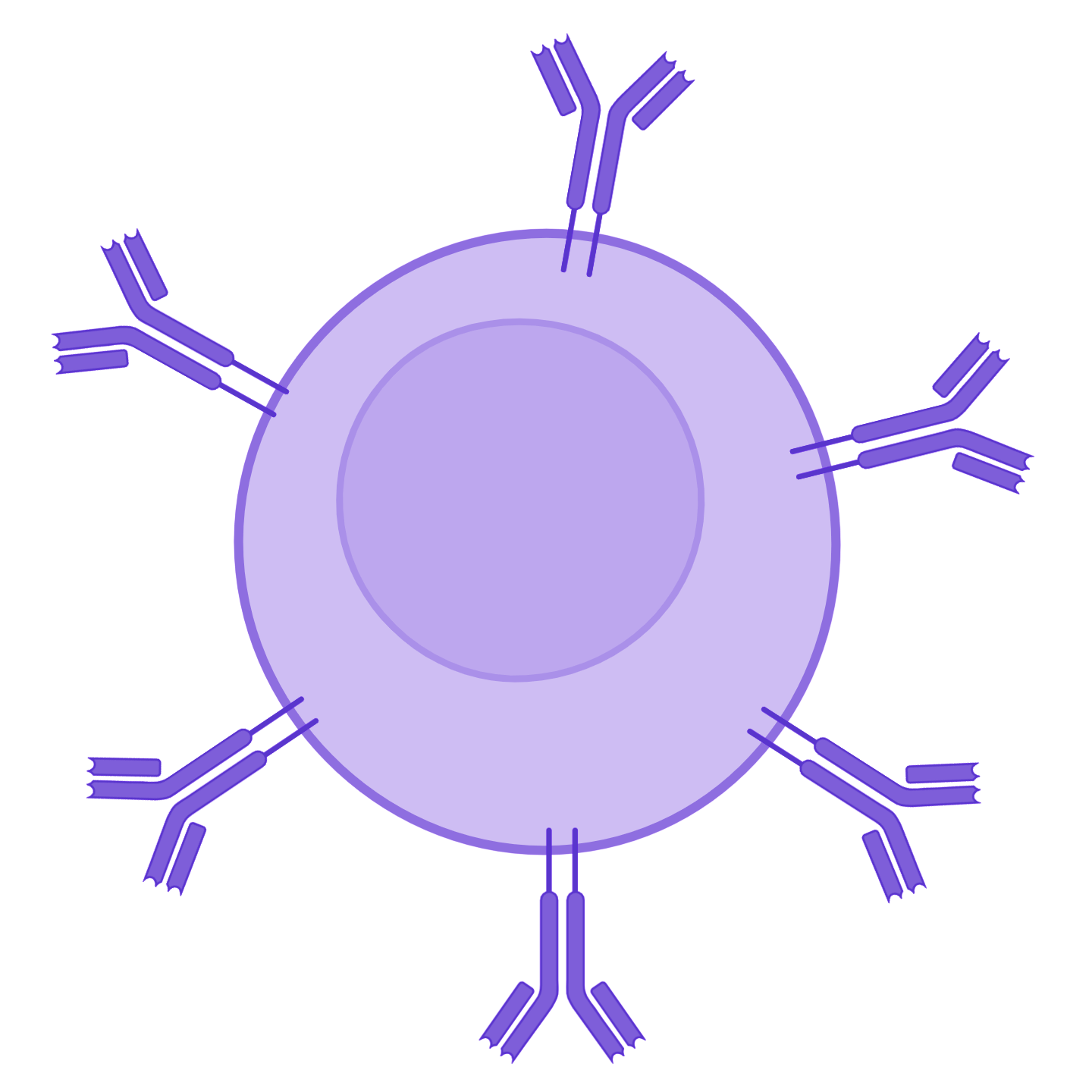
They are looking for the antigen that could be recognised by their BCR. If a B cell recognises a native antigen corresponding to its BCR, this constitutes its first activation signal.
On the other hand, if the BCR of the B cell does not recognize any antigen, then the lymphocyte leaves the secondary lymphoid organ (via the lymphatic efferent duct in the lymph node, via the splenic vein for the spleen) to repeat this operation in another lymphoid organ
How is this done?
Thymus-dependent activation of a B cell requires two signals. This mode of activation affects the vast majority of protein antigens, including those involved in organ transplantation. The activation is called conventional, and involves B cells to allow them to ultimately differentiate:
- either into short-lived plasmablasts producing IgM with low affinity for the antigen. This is the primary immune response;
- or into B cells hat are founders of germinal centres, which will differentiate into memory B cells or long-lived plasma cells. This is the secondary immune response.
Signal 1
Recognition of the native antigen by the BCR of the naive B cell, then internalisation.
Ensures the specificity of B-cell activation
- soluble;
- of a circulating immune complex;
- of immune complex attached to follicular dendritic cells or macrophages present in secondary lymphoid organs.
The engagement of at least two BCRs of the same B cell results in the formation of a bridge necessary for signal transduction by CD79 associated with the BCRs. The downstream pathway is initiated by the phosphorylation of the ITAM motifs of the CD79 chains, which will induce the expression of chemokine receptors on the surface of the B cell, and thus promote the migration of the B cell into the T/B cooperation zone of the secondary lymphoid organs.
In parallel, the antigen is internalised and processed to be presented via a Class II HLA molecule.
Reminder
The B cell is not capable of cross-presentation.
Signal 2
Interaction between the B cell and a previously activated CD4+ T cell.
Reciprocal activation of T and B cells: T/B cooperation
The B cell behaves like a professional antigen-presenting cell by presenting the antigen previously recognised by its BCR, then internalised, via its Class II HLA molecules. The follicular CD4+ T cell previously activated and having migrated into the follicular zone of the lymph node will recognise the complex [peptide – Class II HLA molecule] via its TCR.
Following this interaction, the CD4+ T cell expresses CD28and secretes interleukins, which will allow the B cell to continue its maturation towards transformation into a plasma cell or memory B cell.
Consequences
Interactions between the CD4+ T cell and B cell promote:
- education of germinal centres
The germinal centre is a substructure dedicated to the generation of B cell clones expressing immunoreceptors (BCR) with very high affinity for an antigen.
A germinal centre develops at the expense of the follicular zone, whether in the spleen or in the lymph node. The appearance of a germinal centre signals the transition from a primary follicle to a secondary follicle.
- Somatic hypermutation of the BCR
The B cell clone engaged in the formation of a germinal centre proliferates and undergoes somatic hypermutations of the genes encoding the BCR variable region thanks to an enzyme called AID (Activation-Induced Cytidine Deaminase). The follicular dendritic cells present in the environment present the antigen to them continuously to test the affinity of the new receptor towards the antigen :
- if the new BCR has better affinity, then the B cell survives and differentiates into a plasma cell or a memory B cell;
- if, on the contrary, the BCR has a lower affinity, then the clone degenerates.
- Class switching
B cells initially express only membrane IgM or IgD (see B cell ontogeny). The interaction with the CD4+ T cell, as well as the cytokine environment within the secondary follicle modifies the cellular programming of the B cell (notably the splicing programs), which makes it possible to modify the isotype of the immunoglobulins secreted by the plasma cell (IgM ➔ IgG, IgA or IgE).
- Differentiation of B cells into long-lived plasma cells
The interaction of the CD4+ T cell directs the B cell response towards the genesis of long-lived plasma cells and memory B cells.
Immunosuppressants acting on the activation of B cells
In organ transplantation, the inhibition of B cell activation is mainly achieved indirectly, through the inactivation of helper T cells. Drugs specifically targeting the B cell lineage are anti-CD20 monoclonal antibodies and proteasome inhibitors that deplete B cells and plasma cells, respectively.
What needs to be remembered
B cells emerging from the bone marrow acquired a functional BCR and successfully passed the selection stages. They are therefore mature but naive, until their encounter with the antigen. Activation of B cells is only possible if and only if they receive the two activation signals: recognition of the antigen via their BCR – cooperation with an activated CD4+ T cell. These two stages of activation are absolutely necessary for the transformation of the B cell into a plasma cell synthesising immunoglobulins.
Immunosuppressants used in organ transplantation target the activation of T cells, and therefore indirectly block the activation of B cells, which is impossible in the absence of cooperation with an activated CD4+ T cell.