Humoral rejection is defined histologically by the presence of inflammation of the microvasculatureresponsible for partial or complete occlusion of the glomerular (glomerulitis) and/or peri-tubular (peritubular capillaritis) capillaries. These lesions may be associated with arteritis lesions, acute tubular necrosis or thrombotic microangiopathy lesions.
On the other hand, whatever the lesions, they must be the consequence of an interaction between antibodies and endothelium evidenced by one of these 3 criteria according to the Banff classification :
- Linear C4d deposits in peritubular capillaries
- Significant microvascular inflammation or increased mRNA mRNA
testifying to endothelial activation; - Detection of DSA (Donor-Specific Antibodies) directed against HLA molecules or other alloantigens of the donor.
Actors involved
Mechanism
Where?
In the transplanted organ.
When?
In general, antibody-mediated rejection occurs after the first 3 months of transplantation with the appearance of de novo DSA in the recipient.
In exceptional cases where the recipient has preformed DSA before transplant, antibody-mediated rejection can occur within the first 30 days post-transplant.
How is this done?
In parallel with the allorecognition of the donor’s alloantigens by the recipient’s T cells (see alloreactivity), the recipient’s B cells will also recognise native alloantigens via their BCR. This allorecognition will lead to activation of the B cell, allowing it to interact with a CD4+ T cell previously activated by a dendritic cell (see B cell activation). This interaction allows the maturation of the B cell into a plasma cell secreting immunoglobulins with the same specificity as the BCR, that is to say directed towards the alloantigens of the graft.
In the context of organ transplantation, alloantigens correspond in 90% of cases to specific epitopes of the donor’s HLA molecules.
DSAs bind specifically to donor cells expressing the alloantigens targeted by the antibodies. The endothelial cells of the graft are thus the first to be targeted.
The binding of immunoglobulins to endothelial cells initiates, via their Fc fragment, the two main mechanisms responsible for humoral rejection, namely:
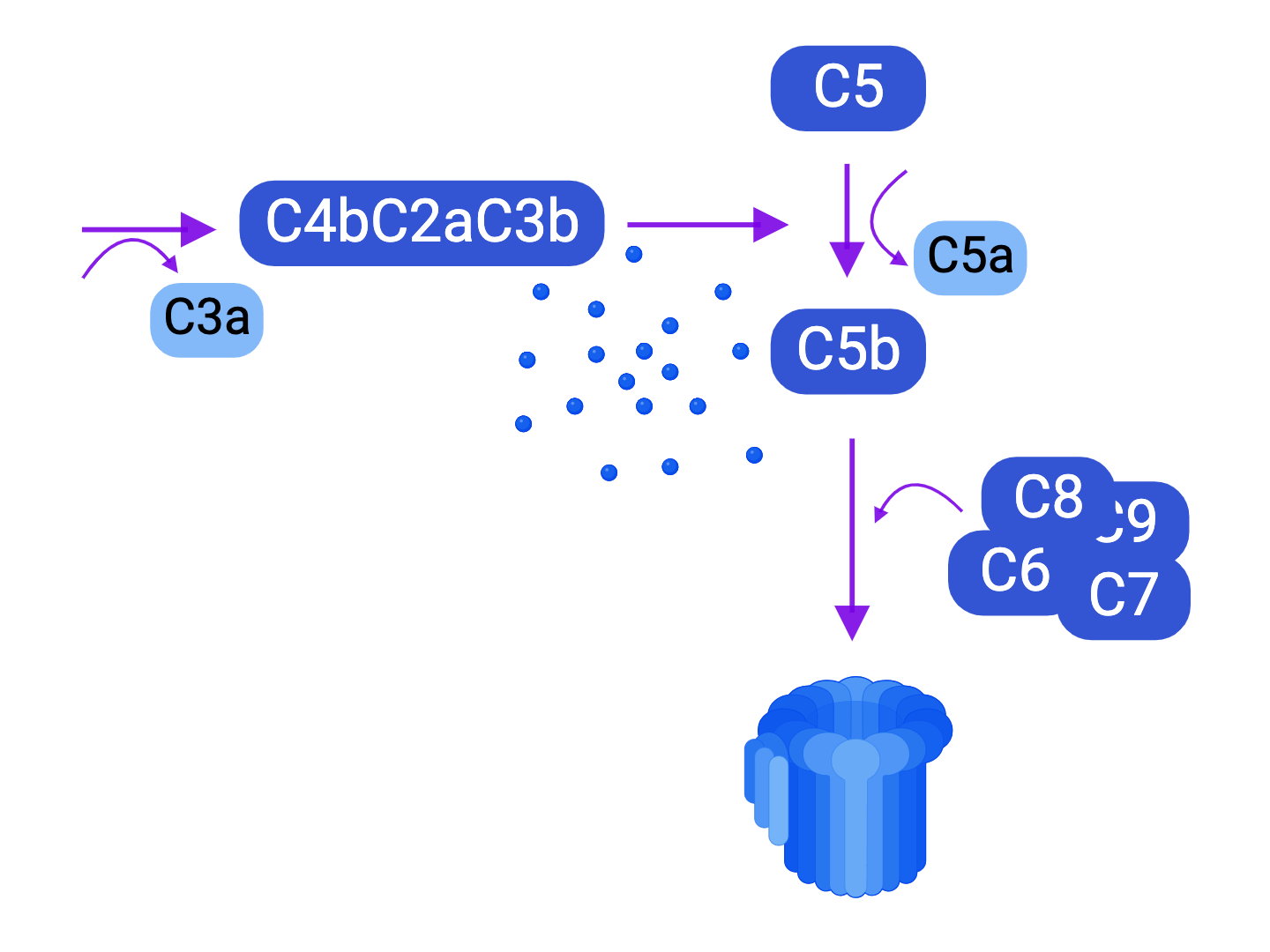
- Activation of the classical complement pathway, and its consequences
- Cytotoxicity by the membrane attack complex
- Recruitment of inflammatory cells
- Facilitation of phagocytosis - Antibody-dependent cytotoxicity (ADCC) mediated by phagocytic cells and NK cells (see immunoglobulins).
Injured endothelial cells secrete von Willebrand factor causing platelet aggregation and adhesion which can lead to thrombus formation. Endothelial damage can also lead to proliferation of endothelial and smooth muscle cells, causing atherosclerosis.
Consequences
- Hyperacute rejection: a few minutes or hours after transplantation, there is irreversible ischemic damage to the transplanted organ and loss of the graft. This type of rejection occurs when DSAs are present in high concentration before transplantation (preformed DSAs).
- Acute rejection: in the first months following transplantation, it is generally associated with the demonstration of de novo DSA.
- Chronic rejection: when the damage/repair cycle is repeated over time in an insidious manner, over several months or years it causes interstitial and perivascular fibrosis and/or atherosclerosis leading to loss of function of the transplanted organ.
Antibody-mediated rejection represents 30% of causes of graft loss. As with cell-mediated rejection, the best strategy to avoid humoral rejection is to prevent it by maintaining immunosuppression.
- Plasma exchanges / immunoadsorption to eliminate DSA.
- Intravenous immunoglobulins (IVIG) which have leiotropic modulatory effects through mechanisms that are still poorly understood (neutralisation of DSA, cytokines, complement, etc.).
- Anti-CD20 monoclonal antibody to deplete plasma cell precursor B cells.
- Proteasome inhibitors to deplete plasma cells.
- Complement inhibitors (monoclonal antibody targeting C5) or plasma C1 esterase inhibitor.
What needs to be remembered
Humoral rejection is the consequence of the synthesis of antibodies directed against donor antigens, also called Donor-Specific Antibodies (DSA). These antibodies will therefore attach to the antigens located on the surface of the donor cells to , on the one hand, initiate the activation of the classic complement pathway, which leads to the formation of the membrane attack complex and the induction of a very pro-inflammatory state; and on the other hand, to make ADCC possible against donor cells. Depending on its temporality, we distinguish between acute, hyperacute, and chronic humoral rejection.
Antibody-mediated rejection represents 30% of causes of graft loss.