The different types of reactivity in the context of transplantation
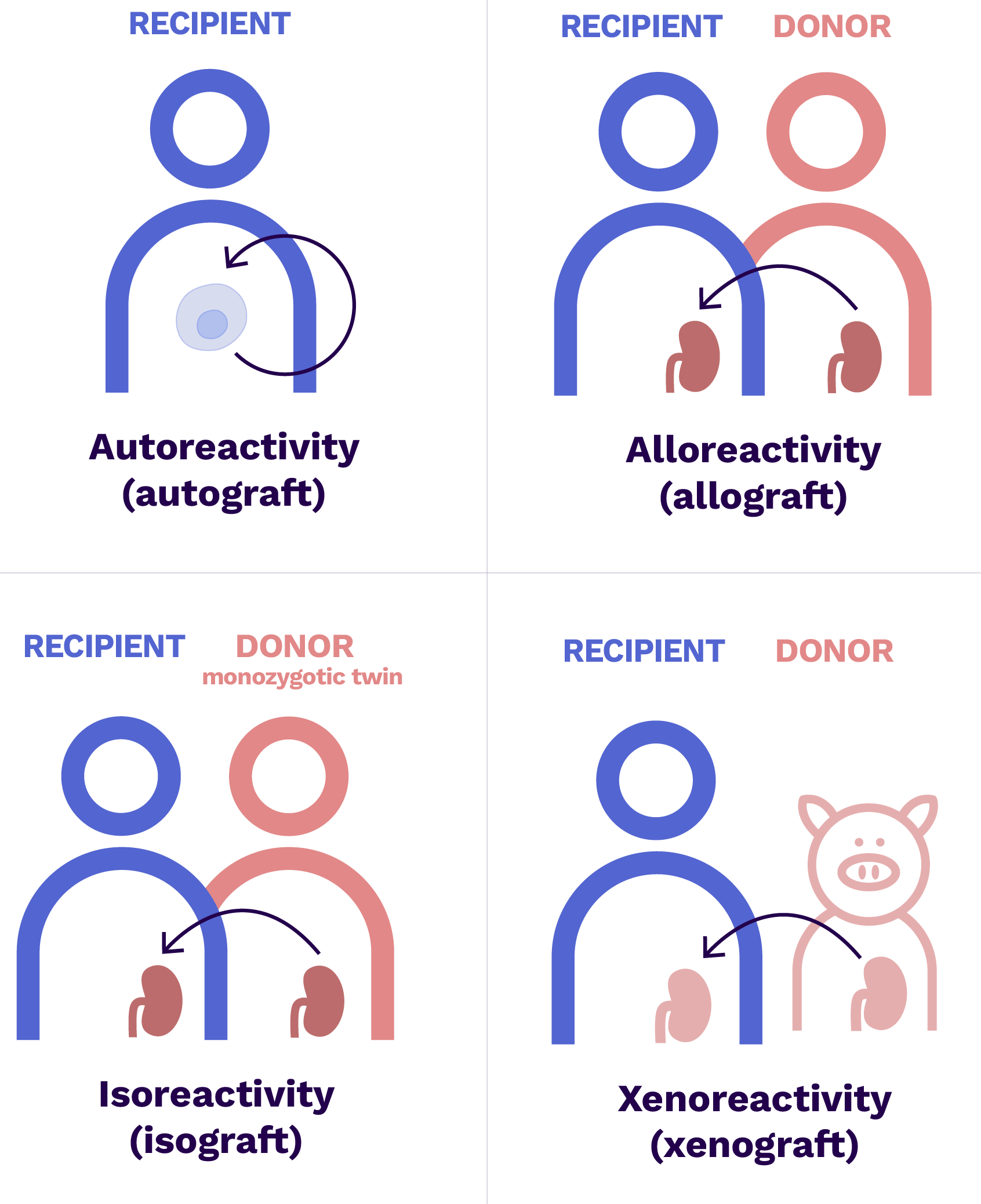
- Autograft = same individual
- Allograft = another genetically different individual of the same species
- Isograft = another individual of the same species but with the same genetic heritage (monozygotic twin)
- Xenograft = individual of a different species
The recipient’s immune system is capable of identifying a cdonor cell as foreign when it presents alloantigens, i.e. antigens present in the donor but absent in the recipient capable of generating an immunological reaction.
Alloantigens are recognised as “non-self” by T and B cells, which are then called alloreactive.
The main alloantigens derive from HLA and ABO molecules : these are the major histocompatibility antigens. But all of the peptides that are present in the donor and absent in the recipient are potential alloantigens against which the recipient’s T and B cells can activate! These other alloantigens are called minor histocompatibility antigens.
Major histocompatibility antigens govern the rules of organ transplantation. Failure to respect these compatibilities systematically leads to rejection in the absence of immunosuppressive treatment.
Studies have identified minor histocompatibility antigens, which are currently not systematically explored in organ transplantation.
Actors involved
Mechanisms
Where?
Allorecognition begins at the graft site. Activation of alloreactive T and B cells takes place in the lymph nodes draining the transplanted organ.
When?
The recipient’s immune system is called upon immediately after the reestablishment of vascularisation of the organ. Without an immunosuppressant, the organ would be rejected within a month of transplantation.
How is this done?
Three allorecognition mechanisms are described in organ transplantation.
In the following explanations, the recipient is always symbolised in bleu and the donor, in red. When both may be involved, the cited component bears both colours.
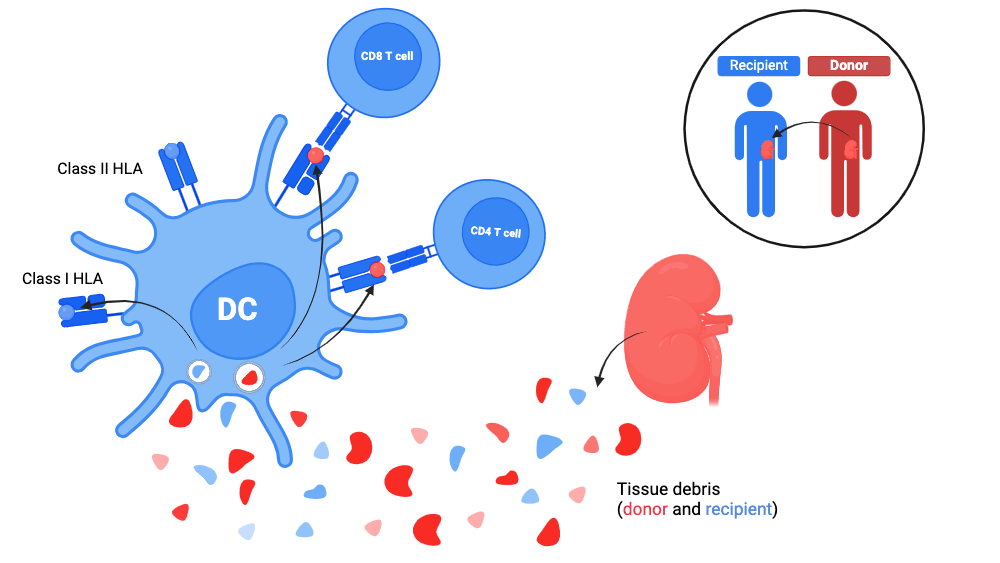
1. Direct allorecognition
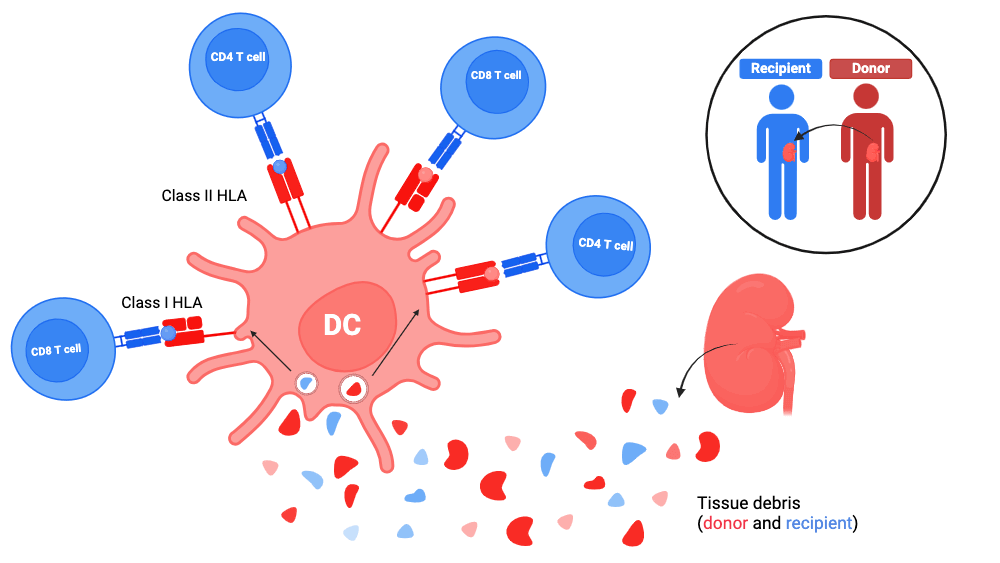
Since dendritic cells are tissue-resident sentinel cells, the transplanted organ must contain donor dendritic cells.
Harvesting the graft and storing it extracorporeally causes an extremely inflammatory state. In this context, the donor’s dendritic cells receive maturation signals, and will be able to move towards the recipient’s lymph nodes draining the graft.
The donor’s dendritic cells thus present to the recipient’s T cells:
- peptides from the donor’s self
and - peptides from the recipient’s tissue debris.
This is done through both:
- Class I HLA molecules from donor dendritic cells
Thanks to traditional priming for the donor peptides and cross-presentation for the recipient peptides (see antigen presentation); - Class II HLA molecules from donor dendritic cells
Thanks to traditional priming for both donor and recipient peptides:
(see antigen presentation ).
To remember
In direct alloreactivity, why can the recipient’s T cells recognise different HLA molecules (donor HLA molecules ) from those that educated them?
- Because during the thymic education of the recipient’s T cells, no thymic cell expresses the donor’s HLA molecules. There is therefore no negative selection (no elimination) of T-cell clones having a strong affinity for foreign HLA molecules ;
- because in the periphery, the [donor HLA - peptide] and [recipient HLA- foreign peptide] complexes can resemble each other and lead to the activation of T cells.
2. Indirect allorecognition
- On the one hand, the recipient’s dendritic cells are attracted to the graft site by the inflammatory microenvironment of the transplantation, in particular thanks to DAMPs and to TLRs.
Once at the graft site, they capture tissue debris and migrate to the recipient’s lymph nodes draining the graft.
- On the other hand, the soluble molecules are brought to the lymph nodes of the recipient by blood or lymphatic route to be taken care of by the dendritic cells resident in the lymph nodes of the recipient.
The donor’s proteins are thus internalised, processed and presented to the recipient’s T cells by the recipient’s dendritic cells via:
- the Class I HLA molecules of the recipient thanks to cross-presentation;
- the Class II HLA molecules of the recipient thanks to traditional priming.
- In indirect allorecognition, these are the donor peptides
- presented by an HLA molecule of the recipient - which are recognised as alloreactive.
The number of alloreactive T cells in indirect allorecognition is therefore similar to that of the number of pathogen-specific T cells (1/1,000,000).
The main allogeneic peptides correspond to peptides derived from the donor’s HLA molecules.
This partly explains why there are plasma cells secreting antibodies against the donor’s HLA molecules (see B cell activation / humoral rejection). - In direct allorecognition, it is the banks of the HLA molecules of the donor that are recognised as alloreactive. The number of alloreactive T cells in the direct allorecognition mechanism is 1,000 to 10,000 times greater than in indirect alloreactivity , and represents 1 to 10% of the total number of T cells.
3. Semi-direct allorecognition
The recipient’s dendritic cells would “capture” [Class I HLA - peptide] complexes from the donor, and present them to the recipient’s CD8+ T lymphocytes.
Consequences
The 3 allorecognition mechanisms – direct, indirect and semi-direct – generate the same reactivity, namely the activation of the recipient’s T cells directed against peptides or HLA molecules specifically expressed by the donor.
Allorecognition thus constitutes the first step of rejection, whether cellular (engagement of the TCR of a CD8+ T cell) and/or humoral (engagement of the TCR of a CD4+ T cell).
What needs to be remembered
| Allo- recognition | Origin of the effector cell (T cell) | Origin of the antigen-presenting cell | Antigens recognised |
|---|---|---|---|
| Direct | Recipient | Donor | HLA donor (+ peptides) |
| Direct? Because the donor’s dendritic cells arrivefirst, and may present “directly” (after migration into the nearest lymph node) to T cells | |||
| Indirect | Recipient | Recipient | Donor peptides |
| Indirect? because the recipient’s dendritic cells arrive “second”, must phagocytise donor antigens, present them, then migrate into the nearest lymph node before presenting them to T-cells | |||
| Semi- direct | Recipient | Recipient | Class I HLA of the donor (+ donor peptides) |
| Semi-direct? because the recipient’s dendritic cells arrive “second” but may present “directly” (after migration into the nearest lymph node) to T cells | |||